
Reducing animal testing is a huge challenge for pharmaceutical companies, and a widely-shared social issue.
Inovotion is committed to reducing the number of animal tests and supporting our customers in this transition.
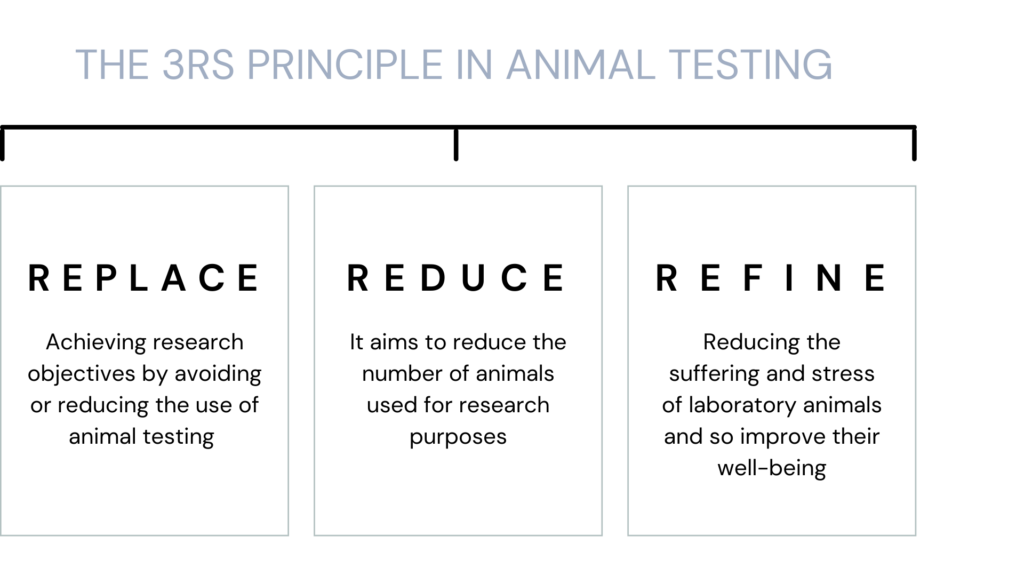
The 3Rs (Replace, Reduce, Refine) objectives and alternative methods to animal experimentation are now highly recommended by regulatory bodies such as the FDA (CDER), EMA, ICH, and by the European Commission
The 3Rs are also a Corporate Social Responsibility (CSR) issue for pharmaceutical companies.
Because Inovotion is working with the chicken embryo and stops the experimentation 2 days before hatching,
it's assays are not considered to be animal experimentation from a regulatory point of view in Europe
(EU directive 2010/63/EU) and the USA.

This implies that authorization for animal experimentation (eg IACUC in the USA) is not needed for the assays.
Thus, all studies carried out with Inovotion’s model are true in vivo assays, without animal testing.
We are “zero animal use” (Replace). As our in vivo data are predictive of results in rodents, we allow our
customers to drastically Reduce and Refine rodent experimentation.


>> LEARN MORE ABOUT OUR TECHNOLOGY
>> DISCOVER OUR ANNOUNCEMENT AT NCRs
>> READ EUROPEAN PARLIMENT RESOLUTION OF 16TH SEPTEMBER 2021